PFO Occlusion System
The nitinol occluder system Nit-Occlud® PFO was specially developed to close congenital PFO heart defects (patent foramen ovale, or persistent foramen ovale). The patented double-umbrella design allows an effective closure of the PFO channel with a very low profile and a minimal amount of material, which reduces thromboembolic risks.
- Benefits
- Details
- handling
- Specifications
-
Benefits
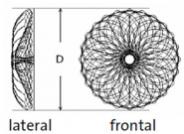
Special design - The occluder consists of a single nitinol wire with a double-layer right atrial disc and a single-layer left atrial disc. The thrombo-embolic risk is reduced by the minimal amount of material used.
Patented single-wire knit - Thanks to the single-wire design, there is no need for the protruding clasps which are usually used. This makes for a very low profile.
Various sizes - The Nit-Occlud® PFO is available in sizes of 20 mm. 26 mm and 30 mm.
Easy to use - The easy-to-use application system was developed specifically for Nit-Occlud® PFO procedures.
Soft atraumatic rim
-
Details
- Single wire-knit double umbrella
- No need for protruding clamps to fixe loose wire ends
- Single-layer left atrial disc in a concave shape
- Diameter of the left atrial disc identical with right atrial disc
- Easy release system
- Repositioning possible at any time prior to release
- Unstressed release of also possible at largest angle
- Pre-mounted 9 F and 10 F systems
- Radiopaque
- MR compatible
Manufacturer
pfm medical mepro gmbh
Am Söterberg 4
66620 Nonnweiler-Otzenhausen, GermanyTech. data
- Woven Nitinol shield with two Dacron membranes
- 120 cm catheter
-
Use
Field of structural heart defects; for treating PFO (patent foramen ovale).
The Nit-Occlud® PFO shield is a permanent implant used for closing PFOs. It is inserted in the PFO using minimumally invasive catheter technology. The shield consists of Nitinol, a shape memory material, which resembles a double shield in its relaxed state.
Dacron, a synthetic material, is incorporated in the right atrial double disc to achieve a high acute closure rate. The left atrial disc is a single disc in order to accelerate the endothelialisation process. Nit-Occlud® shields are available in various sizes to enable a wide range of PFOs to be treated with the best possible results.
The Nit-Occlud® shield system consists of two main components: the shield and a delivery system including a single applicator.
Handling
Diagnostic Measurements
As a first step, a full transesophageal echocardiographic study must be carried out. If possible, all intracardiac findings which are necessary for the procedure should be included in the study.
Selection of the Nit-Occlud® Umbrella
Selection of the umbrella will depend on the distance between the PFO and the aortic root or between the PFO and the junction of the superior vena cava, as measured in the transesophageal echocardiogram (cf. Tab.: Selection of the Nit-Occlud® PFO umbrella and the recommended Mullins sheath).
A 45° curved 9 F Mullins sheath is recommended for the PFO 20 and 26 umbrella systems, and a 45° curved 10 F Mullins sheath for the PFO 30 umbrella system.
Application Range
Indication
Interventional closure of a patent foramen ovale (PFO) after a neurological event (e.g. transient ischemic attack, stroke), in case of decompression illness or migraine. The Nit-Occlud® PFO umbrella system is not intended for the closure of other structural cardiac defects.
Contraindications
Pathological or physical conditions that exclude the implantation of a Nit-Occlud® PFO umbrella:
- Active endocarditis, or active infection at the time of the implantation
- Anatomical or physiological structures that do not permit transesophageal echocardiography
- Atrial fibrillation or atrial flutter
- Concomitant cardiac anomalies requiring an operative procedure
- Hemorrhagic diseases (coagulopathy, tendency to haemolysis)
- Hypersensitivity to contrast medium or nickel
- Potential physical influence on intracardiac or intravascular structures from the occluder
- The required sheaths cannot be passed through the relevant vessels for access to the PFO
- Thrombus in a vessel through which access to the PFO is made
- Thrombus in the vicinity of the implantation site
-
Product specifications
Election of the Nit-Occlud® PFO umbrella
Occluder Type PFO-20, REF 182020 PFO-26, REF 182026 PFO-30, REF 182030 Diameter 20 mm 26 mm 30 mm Distance aortic root-PFO ≥11 mm, <14 mm ≥14 mm, <16 mm ≥16 mm Distance superior vena cava-PFO ≥11 mm, <14 mm ≥14 mm, <16 mm ≥16 mm Recommended Mullins sheath 45° curved 9FF 45° curved 9F 45° curved 10F Auxiliary Imaging Procedures
Closure of the PFO is performed in the cardiac catheterisation laboratory under transesophageal echochardiography and x-ray fluoroscopy.
Ordering Information
REF Diameter Implantation catheter Implantation catheter length PU 182020 20 mm 9 F max. 90 cm 1 182026 26 mm 9 F max. 90 cm 1 182030 30 mm 10 F max. 90 cm 1