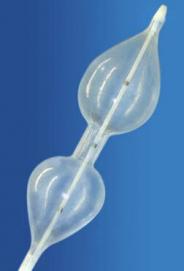
NuCLEUS™ PTV catheter
Recommended for Percutaneous Transluminal Valvuloplasty (PTV) for mitral and aortic positions. The use of this catheter is particularly indicated in stenosis where difficulty in balloon positioning during inflation is experienced.
The NuMED NuCLEUS™ PTV catheter is engineered for maximum steering and tracking. The coaxial shaft design provides enhanced column strength and pushability combined with a flexible distal tip for optimum steerability. The innovative single balloon design facilitates positive positioning while holding the balloon in the correct location prior to and during inflation.
- General
- Specifications
-
Catheter Characteristics
The NuMED NuCLEUS™ PTV catheter is engineered for maximum steering and tracking. The coaxial shaft design provides enhanced column strength and pushability combined with a flexible distal tip for optimum steerability. The innovative single balloon design facilitates positive positioning while holding the balloon in the correct location prior to and during inflation.
Radiopaque Marker
Platinum marker bands facilitate reliable positioning of the balloon and are located at the 'waist' center and beneath the shoulders of the balloon for clear identification under fluoroscopy.
Maximum Trackability
The distal shaft through the balloon is highly flexible for exceptional maneuverability. This, combined with the pushability of the coaxial shaft, provides outstanding tracking performance.
Micro-Thin Non-Compliant Balloon
The NuMED NuCLEUS™ PTV patented design allows for accurate balloon placement. Initial inflation will hold balloon in the desired position, further inflation expands the center of the balloon to effect satisfactory dilatation.
The NuMED NuCLEUS™ PTV balloon is micro-thin for a low deflated profile that maintains tip flexibility. The exceptionally low profile balloon requires the smallest introducer possible. Nominal dimensions are maintained over the entire length of the non-compliant balloon.
-
Product specifications
Balloon
Diameter
(MM)Balloon
Length
(CM)Introducer
Size
(FR)Shaft
Size
(FR)Usable
Length
(CM)Guide
Wire
(Inches)Rated
Burst
(ATM)Catalog
No.10.0 3.0 7 6 110 0.035 9 PVN218 10.0 4.0 7 6 110 0.035 9 PVN219 10.0 5.0 7 6 110 0.035 9 PVN236 10.0 6.0 7 6 110 0.035 9 PVN237 12.0 3.0 7 6 110 0.035 7 PVN220 12.0 3.0 8 6 110 0.035 7 PVN221 12.0 4.0 7 6 110 0.035 7 PVN222 12.0 4.0 8 6 110 0.035 7 PVN223 12.0 5.0 8 6 110 0.035 7 PVN238 12.0 6.0 8 6 110 0.035 7 PVN239 14.0 3.0 9 7 110 0.035 6 PVN224 14.0 4.0 9 7 110 0.035 6 PVN225 14.0 5.0 9 7 110 0.035 6 PVN240 14.0 6.0 9 7 110 0.035 6 PVN241 16.0 3.0 9 7 110 0.035 5 PVN226 16.0 4.0 9 7 110 0.035 5 PVN227 16.0 5.0 9 7 110 0.035 5 PVN242 16.0 6.0 9 7 110 0.035 5 PVN243 18.0 3.0 10 8 110 0.035 4 PVN228 18.0 4.0 10 8 110 0.035 4 PVN229 18.0 5.0 10 8 110 0.035 4 PVN244 18.0 6.0 10 8 110 0.035 4 PVN245 20.0 4.0 12 8 110 0.035 4 PVN230 20.0 5.0 12 8 110 0.035 4 PVN246 20.0 6.0 12 8 110 0.035 4 PVN247 22.0 4.0 12 9 110 0.035 4 PVN231 22.0 5.0 12 9 110 0.035 4 PVN248 22.0 6.0 12 9 110 0.035 4 PVN249 25.0 4.0 12 9 110 0.035 4 PVN232 25.0 5.0 12 9 110 0.035 4 PVN250 25.0 6.0 12 9 110 0.035 4 PVN251 28.0 4.0 12 9 110 0.035 2 PVN233 28.0 4.0 14 9 110 0.035 2 PVN234 28.0 5.0 14 9 110 0.035 2 PVN252 28.0 6.0 14 9 110 0.035 2 PVN253 30.0 4.0 14 9 110 0.035 2 PVN235 30.0 5.0 14 9 110 0.035 2 PVN254 30.0 6.0 14 9 110 0.035 2 PVN255 The devices listed have CE mark approval. Any customer or distributor outside of the EU will be subject to their individual country's regulations in regards to the importation and sale of these products.
This publication could include technical or other inaccuracies or typographical errors. Changes are periodically added to the information herein; these changes will be incorporated in the new editions of the publication. NuMED Incorporated may make improvements and/or changes in the product(s) and/or the program(s) described in this publication at any time.