PTS® Sizing Balloon Catheter
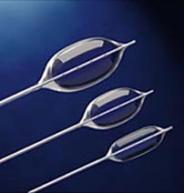
The PTS® Sizing Balloon catheter has been designed for use in patients with cardiovascular defects where accurate measurement of the defect is important to select the appropriately sized occluder device.
- General
- Specifications
-
Catheter Characteristics
The PTS® Sizing Balloon catheter has been designed for use in patients with cardiovascular defects where accurate measurement of the defect is important to select the appropriately sized occluder device.
Radiopaque Marker
Platinum marker bands facilitate reliable positioning of the balloon and calibration for accurately sizing the defect.
Super-Thin Balloon
The PTS® Sizing Balloon catheter is super-thin for a low deflated profile that maintains tip flexibility.
Double Tapered Balloon
The PTS® Sizing Balloon catheter has short tapers at the distal and proximal ends for enhanced transition across the stenosis and post dilatation ease of withdrawal into the introducer.
-
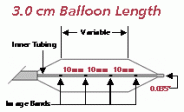
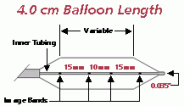
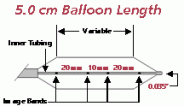
Product specifications
Balloon
Diameter
(MM)Balloon
Length
(CM)Introducer
Size
(FR)Shaft
Size
(FR)Usable
Length
(CM)Guide
Wire
(Inches)Rated
Burst
(ATM)Catalog
No.20.0 3.0 8 8 80 0.035 1.5 PTS203 20.0 3 8 8 110 0.035 1.5 PTS204 25.0 3.0 8 8 80 0.035 1.5 PTS253 25.0 3 8 8 110 0.035 1.5 PTS254 30.0 3.0 8 8 80 0.035 1.0 PTS303 30.0 4.0 8 8 80 0.035 1.0 PTS304 30.0 5.0 8 8 80 0.035 1.0 PTS305 30.0 3 8 8 110 0.035 1.0 PTS306 30.0 4 8 8 110 0.035 1.0 PTS307 30.0 5 8 8 110 0.035 1.0 PTS308 40.0 3.0 9 8 80 0.035 0.5 PTS403 40.0 4.0 9 8 80 0.035 0.5 PTS404 40.0 5.0 9 8 80 0.035 0.5 PTS405 40.0 3 9 8 110 0.035 0.5 PTS406 40.0 4 9 8 110 0.035 0.5 PTS407 40.0 5 9 8 110 0.035 0.5 PTS408 The devices listed have CE mark approval. Any customer or distributor outside of the EU will be subject to their individual country's regulations in regards to the importation and sale of these products.
This publication could include technical or other inaccuracies or typographical errors. Changes are periodically added to the information herein; these changes will be incorporated in the new editions of the publication. NuMED Incorporated may make improvements and/or changes in the product(s) and/or the program(s) described in this publication at any time.