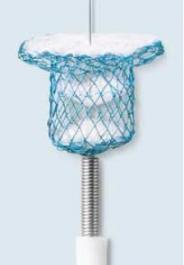
Nit-Occlud PDA-R
PFM medical´s new occlusion system, specifi cally developed for the trans-catheter occlusion of medium to large PDAs (Persistent Ductus Arteriosus). The patented nitinol wire mesh is shaped into a cylindrical plug with a retention disc. Sewn in polyester patches promote endothelialisation and accelerate occlusion.
- General
- Specifications
-
PFM medical´s new occlusion system, specifi cally developed for the trans-catheter occlusion of medium to large PDAs (Persistent Ductus Arteriosus). The patented nitinol wire mesh is shaped into a cylindrical plug with a retention disc. Sewn in polyester patches promote endothelialisation and accelerate occlusion.
Patented single-wire design
The implant is knitted from a single nitinol wire. This gives the distal disc a very low profile with no connecting hubs.
Re-inforced retention disc
The re-inforced retention disc facilitates the implantation. The polyester fabric facing the aorta promotes accelerated endothelialisation.
Membranes for immediate closure
2–4 membranes (depending on the size) are sewn into the implant and one membrane is sewn at the aortic face to ensure immediate closure in the cathlab and to promote endothelialisation.
Flexible adaptive design
Optimal compromise between flexibility and strength, to adapt to various ductus anatomies and to close even larger PDAs.
Safe and simple device release
The unique release mechanism is easy of use, while enabling a soft and tension free release.
-
Product specifications
Permanent implant for the closure of Persistent Ductus Arteriosus (PDA).
REF Stent Disc Length Recommended Introducer
Max L.=90cm160104 7 mm 12 mm 8.5 mm 6F 160105 8.5 mm 14 mm 9.5 mm 6F 160106 10 mm 16 mm 11 mm 7F 160107 11.5 mm 18 mm 12 mm 7F 160108 13 mm 20 mm 13.5 mm 9F